A Double-Blind, Placebo-Controlled Trial of Sibutramine for Olanzapine-Associated Weight Gain
Abstract
OBJECTIVE: Weight gain is commonly observed with olanzapine treatment and can increase the risk for obesity, cardiovascular disease, hypertension, and diabetes mellitus. This study examined the effectiveness of sibutramine, an approved weight loss agent, in overweight and obese subjects taking olanzapine for schizophrenia or schizoaffective disorder. METHOD: Each subject had a DSM-IV diagnosis of schizophrenia or schizoaffective disorder, had been taking a stable dose of olanzapine for at least 4 months, and had a body mass index of ≥30 kg/m2 or ≥27 kg/m2 plus at least one cardiovascular risk factor. In a 12-week double-blind, randomized, placebo-controlled study, 37 subjects received placebo or sibutramine (up to 15 mg/day). For the first 8 weeks all subjects participated in weekly group sessions focused on nutrition and behavioral modification. RESULTS: The sibutramine and placebo groups had no significant baseline differences on age, gender, education, ethnicity, diagnosis, weight, body mass index, and blood pressure. At week 12 the sibutramine group had significantly greater losses than the placebo group in weight (mean=8.3 lb, SD=2.4, versus mean=1.8 lb, SD=1.6), waist circumference, body mass index, and hemoglobin A1c. There were no significant differences on most side effects, although the sibutramine group exhibited a mean increase in systolic blood pressure of 2.1 mm Hg (SD=8.5), and anticholinergic side effects and sleep disturbances were at least twice as common in the sibutramine group. CONCLUSIONS: Sibutramine was an effective and well-tolerated adjunct to behavior modification for weight loss in patients with schizophrenia and schizoaffective disorder being treated with olanzapine.
Although conventional antipsychotic agents significantly reduce symptoms in chronically psychotic patients, a substantial number of patients remain impaired by residual symptoms. In a recently published meta-analysis of the efficacy of second-generation antipsychotics, several second-generation antipsychotics (clozapine, amisulpride, risperidone, and olanzapine) were found to be significantly more efficacious than first-generation antipsychotics (1). Improvements have been noted in positive symptoms, negative symptoms, cognitive deficits, mood, impulse control, excitability, and functional recovery (1). Olanzapine and other atypical antipsychotic agents cause fewer extrapyramidal side effects than conventional neuroleptics and are generally better tolerated. However, the atypical antipsychotic agents are not without their side effects. Psychotropic medications in general and antipsychotics in particular may produce substantial weight gain. Case reports and clinical trials have linked olanzapine with substantial weight gain in some patients (2–6). A meta-analysis estimated that the average weight change for subjects treated with antipsychotic agents over a 10-week period ranged from a reduction of 0.39 kg with molindone to increases of 4.15 kg with olanzapine and 4.45 kg with clozapine (7).
In clinical trials, olanzapine treatment was associated with significant weight increases (greater than 7% of baseline weight) in 32% of patients who were underweight, 18% of normal-weight patients, and 11% of overweight patients (3). In four studies, the mean incidence of weight gain of 7% or greater was 41% in patients treated with olanzapine, compared to 12% of those receiving haloperidol and 3% of those receiving placebo (3). A study of 25 inpatients demonstrated a mean weight gain of 12 lb after 12 weeks of treatment with olanzapine at a mean dose of 13.8 mg/day (5). In another study, 94% of day-treatment patients treated with olanzapine (mean dose, 14.1 mg/day) experienced weight gains of greater than 7%. The mean weight gain was 22.1 lb over a 7-month period and correlated with clinical response (8).
By increasing a patient’s risk of obesity, antipsychotic agents may be placing patients at risk for associated morbidity and mortality (9). Patients who gain more than 10% of their total body weight are at risk for developing hypertension and type 2 diabetes mellitus. The risk for diabetes mellitus is increased approximately twofold in the mildly obese, fivefold in the moderately obese, and tenfold in severely obese persons (10). The duration of obesity is an important determinant of the risk for developing diabetes.
The adverse effects of antipsychotic-induced weight gain may be greatest in men, the elderly, patients with higher body mass indexes at baseline, or patients who experience greater degrees of weight gain (11). Thus, the benefits of atypical antipsychotics, including a decreased rate of mortality from suicide, may be offset by the consequences of antipsychotic-induced weight gain (11). Aside from medical complications, weight gain is also an emotionally distressing side effect that contributes to nonadherence with antipsychotic treatment (12–14). Allison et al. (12) reported that patients treated with antipsychotic medications who gain weight have a reduced quality of life, poorer self-reported general health, and decreased vitality.
While switching to a more weight-neutral atypical antipsychotic agent offers promise in halting or reversing weight gain associated with an antipsychotic agent, many patients and their clinicians are reluctant to risk a worsening or return of psychotic symptoms. As a result, various agents have been proposed as adjunctive treatments to attenuate antipsychotic-induced weight gain. In a 6-week double-blind, placebo-controlled study of 26 inpatients, the addition of reboxetine, a selective norepinephrine reuptake inhibitor, to olanzapine at a dose of 10 mg/day resulted in significantly less weight gain (mean=2.5 kg, SD=2.7, versus mean=5.5 kg, SD=3.1) and significant improvement in the Hamilton Depression Rating Scale score (15). In another study, coadministration of fluoxetine was ineffective in diminishing olanzapine-induced weight gain in first-episode schizophrenia patients (16).
Sibutramine hydrochloride, a weight loss agent affecting both serotonin and norepinephrine reuptake, was introduced into the U.S. market in 1997 (17). The hypophagic effect of sibutramine is thought to be mediated, in part, through activation of the serotonin 5-HT2C receptor (18). Sibutramine has been shown to be an effective and well-tolerated weight loss agent for obesity (19). In a 44-week randomized, placebo-controlled trial, sibutramine produced weight loss and a decrease in waist circumference. Fasting triglyceride levels decreased and levels of high-density lipoprotein (HDL) cholesterol increased; these changes are often associated with weight loss (20). In a 1-year placebo-controlled trial with 485 obese individuals, sibutramine reduced mean weight by 4.8 kg in the 10-mg/day group and 6.1 kg in the 15-mg/day group (17).
In a 16-week double-blind trial, the addition of sibutramine, with behavioral weight counseling, to an ongoing antipsychotic regimen was evaluated in stable overweight or obese outpatients with schizophrenia (21). Twenty-one patients were assigned to either sibutramine (up to 15 mg/day) or placebo in a 2:1 ratio. Nineteen completed at least 4 weeks of double-blind treatment (14 taking sibutramine, five taking placebo), and 11 completed the full 16 weeks. There were no significant differences between groups on mean loss of weight (sibutramine, –8.2 lb; placebo, –9.2 lb) or body mass index (sibutramine, –1.32 kg/m2; placebo, –1.46 kg/m2) in a last-observation-carried-forward analysis. The small number of subjects and high dropout rate may have limited the opportunity to detect differences between groups.
While generally well tolerated, sibutramine can affect heart rate (average increase, 3–4 beats/minute) and blood pressure (average increase, 2 mm Hg) (17). The adverse effects of sibutramine in a phase I trial, in which participants received up to 30 mg/day, included insomnia, rapid heart rate, and diastolic hypertension. For participants receiving up to 20 mg/day, nausea, insomnia, dry mouth, rhinitis, and constipation were noted (18, 19).
We conducted a 12-week double-blind, placebo-controlled trial, adding sibutramine or placebo to the medication regimens of schizophrenia subjects with olanzapine-associated weight gain. We chose olanzapine-treated subjects for this study because weight gain is often the most problematic side effect of this otherwise well-tolerated and effective agent.
Method
Subjects
This 12-week double-blind, placebo-controlled, randomized trial was conducted in the adult outpatient clinic of an urban mental health center. The study was approved by the institutional review board of the Massachusetts Department of Mental Health. Subjects were assessed by the investigators for capacity to provide informed consent. All participants received a full explanation of the nature of the study and were required to take a quiz to ensure their understanding of the study. After providing written informed consent, each subject underwent a diagnostic evaluation by a research psychiatrist (D.C.H.) using the Structured Clinical Interview for DSM-IV (22).
Olanzapine-treated patients with a history of weight gain associated with the drug and with the diagnosis of schizophrenia or schizoaffective disorder were recruited for the study. Patients were included in the study if they met the following criteria: age of 18–65 years, well-established compliance with outpatient medication regimens, stable dose of olanzapine for at least 4 months, and no exposure to tricyclic, selective serotonin reuptake inhibitor (SSRI), or monoamine oxidase inhibitor antidepressants for 1 month. Additionally, the patients were required to have a body mass index of ≥30 kg/m2 or a body mass index of 27 kg/m2 plus another risk factor for cardiovascular disease (hypertension, lipid abnormality, diabetes mellitus).
Subjects were excluded from the study if they were unable to provide informed consent, had current substance abuse or significant medical illness (including hepatic or renal disease), had untreated hypertension, had a history of intolerance of sibutramine, were pregnant or breast-feeding, were receiving treatment with agents that induce weight loss, had a history of glaucoma, had heart disease or an abnormal electrocardiogram, or were being treated with antimigraine agents containing serotonin agonists. The patient’s clinic chart was reviewed, the preolanzapine weight was recorded, and the patient’s clinician was consulted to validate the diagnostic assessment. The subjects’ prestudy medication regimens were maintained throughout the 12-week trial. After completing baseline assessments, the subjects were randomly assigned by a research pharmacist to sibutramine and placebo.
Procedure
Each subject was given a 1-week supply of 5-mg sibutramine capsules or identical-appearing placebo capsules and instructed to take two capsules daily. Research psychiatrists (D.C.H., D.C.G., P.M.L.) were allowed to decrease the dose of the study medication to one capsule a day, as indicated for intolerable side effects. After 4 weeks, the dose was increased to three capsules, as tolerated.
For the first 8 weeks, the participants attended a weekly 1-hour group meeting that incorporated weight education, behavior modification, and group support to facilitate healthy dietary changes. Videotapes covered the “Food Guide Pyramid” and “Dietary Guidelines for Americans” from the U.S. Department of Agriculture (23). Weekly goals for meal planning, portion control, food preparation, healthy snacking, and exercise were discussed in the group sessions and followed up in individual meetings with the group leader.
Assessments
Anthropometric, nutrition, and activity assessments
Weight loss was the principal measure of efficacy. Each subject’s body weight and body mass index were measured at baseline, weekly for the first 8 weeks of the study, and at week 12. Waist circumference was measured at baseline, week 8, and week 12. The waist-to-hip ratio was recorded as the narrowest waist measurement relative to the widest hip circumference to provide information regarding body fat distribution (24–26). The percentage of body fat was calculated from skinfold measurements of the biceps, triceps, suprailiac region, and subscapular region (27, 28).
Overall behavior was assessed with a survey of food intake and dietary habits weekly for the first 8 weeks and at week 12 or study end. The subjects’ adherence to and success with individual dietary goals were assessed weekly for the first 8 weeks of the study in the behavioral nutrition group meetings and at termination, by using a 4-day food record. The food frequency questionnaire was administered at baseline and week 12. Energy and nutrient intake was analyzed by using an extensive nutrient database (Minnesota Nutrient Data System) (29, 30).
A quantitative activity questionnaire, the Modifiable Activity Questionnaire (31), was used to assess both leisure and occupational activity components. The questionnaire is administered by an interviewer and assesses activity for the past 12 months. The Positive and Negative Syndrome Scale (32, 33) was administered at baseline and biweekly for the first 8 weeks and at week 12. The Scale for the Assessment of Negative Symptoms (SANS) (34) and the Global Assessment Scale (GAS) (35) were administered at baseline, week 4, week 8, and week 12 or termination. Extrapyramidal side effects were assessed with the Hillside Akathisia Scale (36) and Abnormal Involuntary Movements Scale (AIMS) (37) at baseline, week 4, week 8, and week 12 or termination from the study.
Laboratory analysis
A certified central laboratory performed all analyses. Plasma concentrations of triglycerides, cholesterol, HDL, and low-density lipoprotein (LDL) were measured at baseline and at the end of weeks 4 and 12. Total plasma cholesterol and triglyceride levels were measured enzymatically (38), and the HDL cholesterol fraction was measured after precipitation of low-density and very-low-density lipoproteins with dextran sulfate-magnesium (39). LDL cholesterol values were estimated indirectly for participants with plasma triglyceride levels less than 4.52 mmol/liter (40). Safety laboratory tests, including complete blood count, creatinine, and liver function tests, based on standard hematological and clinical chemistry values, were performed at baseline, week 4, and week 12. The hemoglobin A1c level was measured by high-performance liquid chromatography after overnight dialysis against normal saline to remove the labile fraction (41). Olanzapine serum levels were measured at baseline and week 4 and were analyzed by using gas chromatography.
Statistical Analysis
The data were analyzed by the Biostatistics Center at the Massachusetts General Hospital and the Schizophrenia Program by using the SAS statistical package, version 8.2 (SAS, Cary, N.C.). All statistical tests were two-tailed, and significance was determined at the 0.05 level. Student’s t tests, Fisher’s exact tests, and Pearson’s correlations were used as appropriate. An intent-to-treat analysis was performed, and all available data were analyzed. For all outcome measures, including body weight, body mass index, laboratory assessments, and clinical rating scale scores, we analyzed the change from baseline by using a repeated-measures analysis of covariance, controlling for baseline.
A sequential testing procedure was used, and the difference at 12 weeks was tested first. The week 8 difference was tested only if the week 12 difference was significant, and the week 4 difference was tested only if the week 8 difference was significant.
To assess the possible correlations of baseline covariates with changes in weight and body mass index in the sibutramine-treated group, the slopes of weight and body mass index over time were calculated for each subject. Analyses of variance were used to test the associations of the slopes with categorical baseline covariates, while Pearson correlations were used to test the associations with continuous baseline covariates.
Results
Enrollment and Demographic Characteristics
Fifty subjects were screened, and 43 were enrolled (Figure 1). Six of these subjects were not assigned to treatments for the following reasons: abnormal ECG (N=1), lack of commitment to the study (N=4), and loss of contact (N=1). Thirty-seven subjects were randomly assigned to either sibutramine (N=19) or placebo (N=18). In all, six subjects (16%) did not complete the study. The proportions of patients who withdrew from the study were similar for sibutramine (16%) and placebo (17%). The reasons for dropping out of the study are summarized in Figure 1.
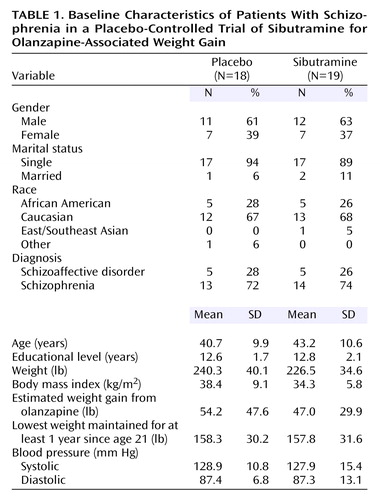
The demographic data are summarized in Table 1. There were no significant differences between the two groups with regard to gender, age, race or ethnicity, marital status, and diagnosis. Weight, body mass index, estimated weight gain from olanzapine, lowest weight since the age of 21, and systolic and diastolic blood pressures did not differ significantly between the sibutramine group and the placebo group at baseline.
Anthropometric and Laboratory Assessments
Table 2 shows the change in weight for both groups. No significant difference in weight loss was found between the two groups at weeks 4 and 8. However, at week 12 the sibutramine group exhibited significantly greater weight loss than the placebo group (t=–2.73, df=52, p=0.009) (Figure 2). Reduction of body mass index was significantly greater for the sibutramine group than for the placebo group at week 8 (t=–2.47, df=51, p=0.02) and week 12 (t=–3.88, df=51, p=0.0003) (Figure 2). In addition, the decrease in waist measurement (t=–3.22, df=25, p<0.004) and the increase in waist-hip ratio (t=2.93, df=25, p=0.007) were significantly greater at week 12 in the sibutramine group than in the placebo group. Changes in measurements of abdominal circumference, neck circumference, and percentage body fat did not differ between groups. Changes in body mass index and weight did not correlate with age, dose of olanzapine, historic lowest weight, baseline weight, weight gain with olanzapine, age at onset of schizophrenia, or educational level.
Throughout the study, there were few clinically significant changes in hematological and biochemical laboratory parameters (Table 3). However, the mean decrease in hemoglobin A1c was significantly greater in the sibutramine group at week 12 (t=–2.93, df=20, p=0.008), with the baseline level controlled, suggesting an improvement in glucose metabolism. There were no differences between the groups at week 12 in measurements of random glucose, uric acid, cortisol, liver functioning, blood urea nitrogen, creatinine, thyroid functioning, or lipids. Additionally, olanzapine blood levels did not change significantly in either group during the course of the study.
Clinical Psychiatric Ratings
Overall, no significant differences between groups were found on the Positive and Negative Syndrome Scale total score and subscale scores, except at week 12, when the placebo group had a lower score than the sibutramine group on the negative symptoms subscale (t=2.04, df=43, p=0.05). However, the difference in negative symptoms was not evident on the SANS total score. There were no differences between the groups with respect to the AIMS, the GAS, and the Hillside Akathisia Scale (Table 4).
Dietary Intake
The food frequency questionnaire and the 4-day food record revealed few differences between the two groups in dietary intake from baseline to week 12 (Table 5). Although not statistically significant, both groups demonstrated reductions in total calories. The sibutramine group showed a significant decrease in total grams of polyunsaturated fatty acids at week 12 (t=–2.31, df=13, p=0.04) and in the percentage of calories from polyunsaturated fatty acids at week 12 (t=–2.42, df=13, p=0.03).
Although the difference was not statistically significant, nighttime snacking decreased in the sibutramine group, and both groups reported reductions in “overeating” that persisted throughout the study. Additionally, both groups reported reductions in eating “empty calorie” foods (cookies, chips, ice cream, french fries, cake, and pie), total calories, calories from fat, calories from sweets, fat servings, and intake (grams) of carbohydrates, total fat, and saturated fat. The percentage of calories from protein at week 12, with the baseline level controlled, was significantly increased in the placebo group (t=–2.29, df=21, p=0.03). Although not statistically significant, the sibutramine group also reported a reduction in meat servings. Vegetable servings increased significantly, however, when the analysis controlled for baseline, in the placebo group (t=–2.13, df=21, p=0.05).
Adverse Events
Diastolic blood pressure, heart rate, and most ECG variables did not differ significantly between groups throughout the study. Systolic blood pressure increased significantly in the sibutramine group at week 12 (t=2.23, df=51, p=0.04). Dry mouth, constipation, blurred vision, excessive thirst (anticholinergic side effects), interrupted sleep, and shortened sleep increased in more than 5% of the subjects in the sibutramine group, and increases in these conditions occurred in at least twice as many patients in the sibutramine group as in the placebo group. On the other hand, dizziness increased to a greater degree in the placebo group (Table 6). No subjects were withdrawn from the study because of adverse events.
Three-Month Follow-Up
Ten placebo subjects and 12 sibutramine subjects returned 3 months after the end of the study for a follow-up assessment. While body mass index, weight, waist circumference, and waist-hip ratio had decreased significantly at week 12, the change from baseline of the sibutramine group was not statistically different from that of the placebo group for waist circumference (sibutramine: mean=–5.3 cm, SD=8.0; placebo: mean=2.3 cm, SD=16.7), waist-hip ratio (sibutramine: mean=0.03, SD=0.05; placebo: mean=–0.1, SD=0.2), weight (sibutramine: mean=–5.7 lb, SD=14.9; placebo: mean=0.3 lb, SD=15.8), or body mass index (sibutramine: mean=–0.2 kg/m2, SD=2.9; placebo: mean=0.04 kg/m2, SD=2.5) at the time of the 3-month follow-up.
Discussion
In this randomized trial, subjects who received sibutramine lost significantly more weight during 12 weeks of therapy than subjects who received placebo. The results are consistent with those from previous studies of sibutramine in obese patients. In this study, sibutramine, combined with group and individual behavioral nutrition counseling, was effective for weight loss in overweight and obese olanzapine-treated subjects over a 12-week period. The subjects showed significantly greater weight loss than those taking placebo, while also reducing body mass index, waist circumference, and the waist-hip ratio; these changes represent a substantial reduction in cardiovascular risk factors. There was a significant improvement in hemoglobin A1C in the sibutramine group, suggesting, as expected, that weight loss in this group had a positive impact on glucose metabolism. Weight loss did not result in improvements on any lipid measurement, suggesting that other mechanisms are responsible for olanzapine-associated hyperlipidemia.
The reductions in weight, body mass index, waist circumference, and waist-hip ratio were not sustained at 3 months following the study completion. The subjects who received sibutramine gained weight and returned to near their baseline weight. This suggests that, as expected, the benefits of sibutramine do not continue after discontinuation of the drug.
In reversing olanzapine-associated weight gain, sibutramine may provide a clue as to the mechanism of olanzapine’s metabolic effects. Sibutramine acts as an agonist at the 5-HT2C receptor, and a polymorphism of the promoter region of the serotonin 5-HT2C receptor gene has been associated with antipsychotic-induced weight gain (42). Among 32 Chinese patients with first-episode schizophrenia, the 10 who had the –759C/T polymorphism showed significantly less weight gain with clozapine than those without this allele. Olanzapine acts as an antagonist at the 5-HT2C receptor. It is possible that sibutramine’s competitive agonist effect at the 5-HT2C receptor decreases an olanzapine-induced increase in appetite (43).
Following a physical examination, baseline safety assessments, and an ECG, sibutramine appeared to be safe and effective at a dose of 10 or 15 mg/day in overweight and obese schizophrenia subjects. Sibutramine was well tolerated, and no serious adverse events occurred. There were no changes in the results of safety assessments, including renal, hepatic, and thyroid tests. Olanzapine blood levels were not altered. While weight loss is usually associated with a decrease in blood pressure, we observed a mild increase in systolic blood pressure. Typical sibutramine-related side effects, including dry mouth and constipation, were observed. However, the dropout rates in the two groups were similar.
Sibutramine did not worsen psychotic symptoms. Whereas the negative symptoms subscore on the Positive and Negative Syndrome Scale decreased significantly more in the placebo group than in the sibutramine group, changes in negative symptoms were not evident on the SANS for either group.
Limitations
The results of this study may not be generalizable to other populations, particularly in the absence of a nutritional counseling program. Sibutramine was not administered to patients taking other serotonergic agents, such as SSRIs and migraine medications. Sibutramine should be used with caution in patients treated with SSRIs, as the risk of serotonin syndrome may be increased. It is also not clear if sibutramine would be an effective weight loss agent in patients treated with other medications that cause significant weight gain, such as clozapine. Additionally, this clinical trial was short (12 weeks), and it is not clear whether the observed weight reductions can be maintained with long-term use of sibutramine.
Conclusions
Sibutramine treatment improved several health status markers that are predictive of cardiovascular disease. This degree of weight loss in obese schizophrenia patients could, if sustained, substantially reduce morbidity and mortality. Sibutramine appeared to be a safe and effective weight loss agent in this study group. Clinicians should consult with primary care clinicians to determine the safety and necessity of sibutramine for individual patients. Additional studies are necessary to establish the long-term weight loss effectiveness and safety in schizophrenia patients whose weight gain from antipsychotic medications jeopardizes their cardiovascular health.
 |
 |
 |
 |
 |
 |
Received Feb. 8, 2004; revision received April 5, 2004; accepted May 14, 2004. From the Schizophrenia Program, Weight Center, Endocrine Unit, and Biostatistics Center, Massachusetts General Hospital, Boston; and the Departments of Psychiatry and Medicine, Harvard Medical School, Boston. Address correspondence and reprint requests to Dr. Henderson, Freedom Trail Clinic, 25 Staniford St., Boston, MA 02114; [email protected] (e-mail). Supported by a Young Investigator Award to Dr. Henderson from the National Alliance for Research on Schizophrenia and Depression and by an investigator-initiated independent research grant from Eli Lilly and Co. Drug and placebo were provided by Knoll Pharmaceuticals (Abbott Laboratories).
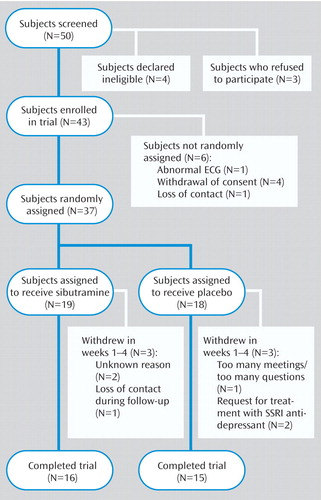
Figure 1. Patients Who Entered and Completed a 12-Week Placebo-Controlled Trial of Sibutramine for Olanzapine-Associated Weight Gain
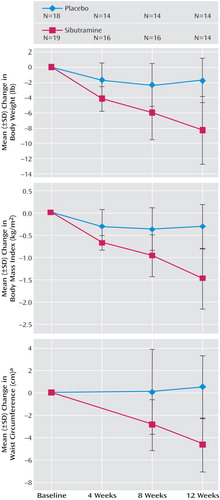
Figure 2. Change in Body Weight Among Patients in a Placebo-Controlled Trial of Sibutramine for Olanzapine-Associated Weight Gain
aNumbers of subjects differ from those shown because of varying patterns of missing data.
1. Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003; 60:553–564Crossref, Medline, Google Scholar
2. Beasley CM Jr, Hamilton SH, Crawford AM, Dellva MA, Tollefson GD, Tran PV, Blin O, Beuzen JN: Olanzapine versus haloperidol: acute phase results of the International Double-Blind Olanzapine Trial. Eur Neuropsychopharmacol 1997; 7:125–137Crossref, Medline, Google Scholar
3. Beasley CM Jr, Tollefson GD, Tran PV: Safety of olanzapine. J Clin Psychiatry 1997; 58(suppl 10):13–17Google Scholar
4. Baptista T, Beaulieu S: Body weight gain, insulin, and leptin in olanzapine-treated patients. J Clin Psychiatry 2001; 62:902–904Crossref, Medline, Google Scholar
5. Osser DN, Najarian DM, Dufresne RL: Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry 1999; 60:767–770Crossref, Medline, Google Scholar
6. Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR: Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999; 60:358–363Crossref, Medline, Google Scholar
7. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Abstract, Google Scholar
8. Gupta S, Droney T, Al-Samarrai S, Keller P, Frank B: Olanzapine: weight gain and therapeutic efficacy. J Clin Psychopharmacol 1999; 19:273–275Crossref, Medline, Google Scholar
9. Pi-Sunyer FX: The medical risks of obesity. Obes Surg 2002; 12(suppl 1):6S-11SGoogle Scholar
10. Pi-Sunyer FX: Medical hazards of obesity. Ann Intern Med 1993; 119(7, part 2):655–660Google Scholar
11. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshminarayanan M, Casey DE, Allison DB: Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001; 101:277–288Crossref, Medline, Google Scholar
12. Allison DB, Mackell JA, McDonnell DD: The impact of weight gain on quality of life among persons with schizophrenia. Psychiatr Serv 2003; 54:565–567Link, Google Scholar
13. Perkins DO: Adherence to antipsychotic medications. J Clin Psychiatry 1999; 60(suppl 21):25–30Google Scholar
14. Perkins DO: Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002; 63:1121–1128Crossref, Medline, Google Scholar
15. Poyurovsky M, Isaacs I, Fuchs C, Schneidman M, Faragian S, Weizman R, Weizman A: Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. Am J Psychiatry 2003; 160:297–302Link, Google Scholar
16. Poyurovsky M, Pashinian A, Gil-Ad I, Maayan R, Schneidman M, Fuchs C, Weizman A: Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry 2002; 159:1058–1060Link, Google Scholar
17. Glazer G: Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety. Arch Intern Med 2001; 161:1814–1824Crossref, Medline, Google Scholar
18. Finer N: Present and future pharmacological approaches. Br Med Bull 1997; 52:409–432Crossref, Google Scholar
19. Weintraub M, Rubio A, Golik A, Byrne L, Scheinbaum M: Sibutramine in weight control: a dose-ranging, efficacy study. Clin Pharmacol Ther 1991; 50:330–337Crossref, Medline, Google Scholar
20. Wirth A, Krause J: Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001; 286:1331–1339Crossref, Medline, Google Scholar
21. Radulovic L, Weiden P, Allison DB: A double-blind, placebo-controlled trial of adjunctive sibutramine for obesity in patients with schizophrenia, in Proceedings of the 2002 Meeting of the New Clinical Drug Evaluation Unit. Rockville, Md, NCDEU, 2002, pp 1–15Google Scholar
22. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
23. Nutrition and Your Health: Dietary Guidelines for Americans, 4th ed: Home and Garden Bulletin 232. Washington, DC, US Department of Agriculture and US Department of Health and Human Services, Dec 1995Google Scholar
24. Lohman TG: Exercise training and body composition in childhood. Can J Sport Sci 1992; 17:284–287Medline, Google Scholar
25. Jensen MD: Research techniques for body composition assessment. J Am Diet Assoc 1992; 92:454–460Medline, Google Scholar
26. Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW: Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54:254–260Crossref, Medline, Google Scholar
27. Durnin JV, Womersley J: Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974; 32:77–97Crossref, Medline, Google Scholar
28. Lange TG, Roche AF, Martorell R: Anthropometric Standardization Reference Manual. Champaign, Ill, Human Kinetics Books, 1988Google Scholar
29. Willet W: Nutritional Epidemiology. New York, Oxford University Press, 1998Google Scholar
30. Thompson FE, Byers T: Dietary assessment resource manual. J Nutr 1994; 124(11 suppl):2245S-2317SGoogle Scholar
31. Kriska AM, Caspersen CJ: Introduction to the collection of physical activity questionnaires, in A Collection of Physical Activity Questionnaires for Health-Related Research. Edited by Kriska AM, Caspersen CJ. Med Sci Sports Exerc 29(suppl 6):S5-S9, S73-S78, 1997Google Scholar
32. Kay SR, Singh MM: The positive-negative distinction in drug-free schizophrenic patients: stability, response to neuroleptics, and prognostic significance. Arch Gen Psychiatry 1989; 46:711–718Crossref, Medline, Google Scholar
33. Kay SR, Opler LA, Lindenmayer JP: Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenics. Psychiatry Res 1988; 23:99–110Crossref, Medline, Google Scholar
34. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
35. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
36. Fleischhacker WW, Bergmann KJ, Perovich R, Pestreich LK, Borenstein M, Lieberman JA, Kane JM: The Hillside Akathisia Scale: a new rating instrument for neuroleptic-induced akathisia. Psychopharmacol Bull 1989; 25:222–226Medline, Google Scholar
37. Munetz MR, Benjamin S: How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry 1988; 39:1172–1177Abstract, Google Scholar
38. McNamara J, Schaefer E: Automated enzymatic standardized lipid analyses for plasma lipoprotein fractions. Clin Chim Acta 1987; 166:1–8Crossref, Medline, Google Scholar
39. Warnick G, Benderson J, Albers J: Dextran sulfate-Mg2+ precipitation procedure for quantization of high-density-lipoprotein cholesterol. Clin Chem 1982; 28:1379–1388Medline, Google Scholar
40. Friedewald W, Levy R, Fredrickson D: Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502Medline, Google Scholar
41. Nathan DM: Labile glycosylated hemoglobin contributes to hemoglobin A1 as measured by liquid chromatography or electrophoresis. Clin Chem 1981; 27:1261–1263Medline, Google Scholar
42. Reynolds GP, Zhang ZJ, Zhang XB: Polymorphism of the promoter region of the serotonin 5-HT2C receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679Link, Google Scholar
43. Tarazi FI, Zhang K, Baldessarini RJ: Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology (Berl) 2002; 161:263–270Crossref, Medline, Google Scholar



